The Cognitive Function Of Wistar Rats Subjected To Cafeteria Diet And To Chronic Stress
da Silva WAM, Mendes BO, Guimarães ATB, e Silva BC, Ferreira RO, de Almeida SF, Rabelo LM, Estrela DC, de Souza JM and Malafaia G
DOI10.21767/2471-8203.100012
Malafaia G1,2*, da Silva WAM3, Mendes BO3, Guimarães ATB3, e Silva BC3, Ferreira RO3, de Almeida SF3, Rabelo LM3, Estrela DC1 and de Souza JM3
1Programa de Pós-Graduação em Biodiversidade Animal, Universidade Federal de Goiás – Câmpus Samambaia, Brazil
2Departamento de Ciências Biológicas, Laboratório de Pesquisas Biológicas, Instituto Federal Goiano – Câmpus Urutaí, GO, Brazil
3Laboratório de Pesquisas Biológicas, Instituto Federal Goiano – Câmpus Urutaí, GO, Brazil
- *Corresponding Author:
- Malafaia G
Departamento de Ciências Biológicas
Laboratório de Pesquisas Biológicas
Instituto Federal Goiano– Câmpus Urutaí
GO, Brazil
Tel: 75790000
E-mail: guilhermeifgoiano@gmail.com
Received date: May 04, 2016; Accepted date: May 23, 2016; Published date: May 28, 2016
Citation: da Silva WAM (2016) The Cognitive Function of Wistar Rats Subjected to Cafeteria Diet and to Chronic Stress. J Obes Eat Disord 2:12.doi:10.21767/2471-8203.100012
Copyright: © 2016, Malafaia G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of the current study is to evaluate the effect of chronic stress of obese or non-obese female rats on the cognitive function of animals. The animals were distributed in the following groups: standard diet (Std), Std+stress, cafeteria diet (Cafe) and Cafe+stress. The animals in the Std groups were fed standard rodent diet. The animals in the Cafe groups received palatable processed high-calorie foods, cola-type beverages, water and water with sucrose ad libitum, in addition to the standard diet. From the eighth experimental week on, the Std+stress and Cafe+stress groups were subjected to restraint-induced chronic stress model (50 days). Next, the animals were subjected to the object recognition test. The animals in the Std, Std+stress and Cafe+stress groups showed higher new object recognition rate in comparison to that of the familiar object (square Lego toys used during the training session of the object recognition test). Our data also shows memory improvement in the stressed animals (obese and non-obese). We observed higher N object recognition index in the Std+stress and Cafe+stress groups, in comparison to their respective controls. The study supports the hypothesis that moderate and repeated stress improves the memory of obese female Wistar rats.
Keywords
Obesity; Experimental models; Chronic stress; Cognition
Introduction
Obesity has been considered an epidemic and one of the main public health issues worldwide. It is commonly defined as the excessive accumulation of body fat, which leads to other health complications, thereby reducing human life expectancy [1]. Different comorbidities, such as psychological disorders, osteoarthritis, type 2 diabetes mellitus, hypertension, hyperlipidemia, fatty liver, cardiovascular diseases and certain types of cancer, may be associated with obesity [2]. The literature shows that obesity leads to neurological disorders [3]. Obesity may affect the learning ability and cognitive functions of obese middle-aged and young people [4]. Gunstad et al. [5] found that 23.9% of the people with severe obesity (i.e., bariatric surgery candidates) showed learning deficits and that 22.9% of them showed memory deficits. Increased Body Mass Index (BMI) has been associated with low cognitive performance and, apparently, it does not vary due to aging. This fact provides strong evidence that the adiposity effects on cognition go beyond the medical comorbidities (for example, hypertension, type 2 diabetes mellitus, sleep apnea, among others). Different studies show that patients showed memory improvement after the obesity treatment [6,7]. Overall, these results suggest that the cognitive impairment prevails before the bariatric surgery, but it quickly improves after the surgery. Studies conducted on rodents confirmed that obesity impairs cognition [8]. This impairment becomes evident when rodents are required to use the hippocampus and the surrounding cortex in tasks such as the Morris water maze, in which they should use the spatial relationships between the lanes to navigate to a hidden platform [9]. Cognitive deficiencies have also been reported in other behavioral tests, such as T-maze [10], radial maze [11], object recognition test [12], as well as tests whose responses are not necessarily linked to the hippocampus and to the cerebral cortex [13,14].
Stress, which is related to the modern world dynamics, is another health issue affecting millions of people [15]. According to Taylor and Stanton [16], stress may result from a particular condition and/or lifestyle and lead to a wide range of behavioural changes. Among these changes, it is worth highlighting those related to eating habits, which reflect the interaction between the body physiological status and the environmental conditions [17]. The relationship among chronic stress, brain plasticity and cognition is complex. The hippocampus and the amygdala are particularly susceptible to corticosterone-mediated changes, since both structures contain a large number of glucocorticoid receptors [18]. Several types of stressors may be categorized according to their psychogenic factors and related to the psychological or neurological disorders they may cause. It is well known that restraint-induced stress does not involve physical pain, but it can induce stress in animals and result in behavioural, neurochemical and morphological changes [19]. According to Bowman et al. [19], rodents exposed to the restraint-induced stress protocol show negative effects, such as memory deficits, on the central nervous system structures.
Several studies investigated the effects of stress and obesity on organic functions in experimental models, including rats and mice [20]. However, most of these studies separately investigated the effects of obesity and stress. On the other hand, some studies investigated the effects of the association between obesity and chronic stress on experimental models [21,22], but they did not address issues related to cognitive functions [23], involving male Sprague Dawley rats and male Wistar rats [24]. Therefore, the lack of studies linking these two pathological conditions and evaluating its effects on cognitive function is an interesting gap that needs to be further investigated, especially considering that human studies have shown that a stressful life combined with diets rich in fat and carbohydrates exerts adverse effect on human health and well-being. Another interesting aspect lies on the fact that although obesity is most prevalent among women [25], the experimental studies were focused on male animals. Important studies found different results when males and females were evaluated in studies involving obesity [26] and stress [27]. However, only a limited number of studies has been conducted on female rodents, fact that also features a knowledge gap that deserves attention. Regarding obesity, although some studies used female rats in their experiments, their behavior with respect to their cognitive functions has not been the focus of these studies.
Finally, we emphasize that physiological and behavioural data, taken together, suggest that spatial memory can be improved given a moderate level of stress exposure, in nonobese rodents [28]. It has also been suggested that there is an “inverted U” relationship between stress and cognitive function [29], such that a moderate level of glucocorticoids has pro-cognitive effects, whereas too low or too high glucocorticoid levels are detrimental to cognitive processing [30]. Thus, the aim of the current study is to evaluate the effect of chronic stress on obese and non-obese female rats. We questioned whether obesity might affect female Wistar rats’ memory and we used the object recognition test to investigate whether chronic stress in obesity exerts some influence on the cognitive function of animals.
Materials and Methods
Animals and experimental groups
The herein presented study used female Wistar rats coming from matrices of the Central Animal Facility at Federal University of Goiás (UFG) - Campus Samambaia (Goiânia, GO, Brazil) and kept in the Animal Facility of the Biological Research Laboratory at Federal Institute of Goiás (IF Goiano) - Câmpus Urutaí (Urutaí, GO, Brazil). The animals were subjected to normal light/dark cycle, with food and liquids offered ad libitum. The animals were 45 days old (age corresponding to the final puberty stage) and they were distributed in the following experimental groups: standard diet (Std); standard diet+stress (Std+stress); cafeteria diet (Cafe) and cafeteria diet+stress (Cafe+stress). It is noteworthy that the diets began to be offered to the animals when they were 45 days old and that the behavioral test was performed when the animals were young adults. Each experimental group consisted of six animals and two independent experiments were performed, thus total of 12 animals per group. The animals were kept in cages containing at least three animals each. All procedures were approved by the Ethics Committee on Animal Use (CEUA) IF Goiano, GO, Brazil (protocol n. 003/2012).
Experimental design
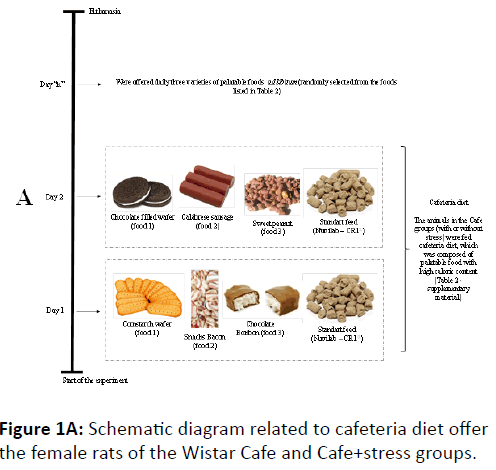
The animals in the Std groups (with or without stress) were fed standard rodent diet (Nuvilab-CR1®) whose nutritional composition is shown in supplementary material (Table 1). The animals in the Cafe groups (with or without stress) were fed cafeteria diet, which was composed of palatable food with high caloric content (Table 2-supplementary material). In order to do so, we used a total of 49 palatable foods, which were close to the variety of highly caloric foods usually consumed by humans.
| Ingredients of the standard feed(1) | |
|---|---|
| Ground whole corn, soybean bran, wheat bran, calcium carbonate, dicalcium phosphate, sodium chloride, mixture of vitamins, minerals and amino acids. | |
| Specifications(1) | Warranty levels per kg of the product (g/kg)(1) |
| Moisture | 125 |
| Casein(2) | 220 |
| Ether extract | 40 |
| Mixture of salts | 90 |
| Fibrous matter | 70 |
| Calcium | 10 |
| Phosphorus | 8 |
| kcal | 2930 |
| Enrichment per kg of the product | |
| Vitamins: Vitamin A 13.000 UI; Vitamin D3 2.000 UI; Vitamin E 34 UI; Vitamin K3 3 mg; Vitamin B1 5 mg; Vitamin B2 6 mg; Vitamin B6 7 mg; Vitamin B12 22 µg; Niacin 60 mg; Calcium pantothenate 20 mg; Folic acid 1 mg; Biotin 0.05 mg; Choline 1900 mg. Minerals: Zinc 60 mg; Copper 10 mg; Iodine 2 mg; Selenium 0.05 mg; Cobalt 1.5 mg; Fluorine 80 mg. Amino acids: Lysine 12 g; Methionine 4,000 mg. Additives: BHT 100 mg. |
|
Table 1: Nutritional information from the rodent standard feed (Nuvilab – CR1) (1) Information obtained from food packaging (Nuvital, 2014) (2) The protein content of casein used was approximately 80%.
It is worth clarifying that the animals in the Cafe and Cafe +stress groups were daily offered three varieties of palatable foods ad libitum (randomly selected) based on the foods listed in Table 2 (supplementary material), in addition to the standard rodent diet. Therefore, the cafeteria diet was daily changed as the food offered to the animals was chosen. Figure 1A schematically illustrates the palatable food supply dynamics and the composition of the cafeteria diet offered to the animals. The animals in the Cafe and Cafe+stress groups received the same palatable food variety, in addition to the standard diet. The Std and Std+stress groups received only standard rodent diet.
| Processed foods and portion (in grams) as regards their nutritional composition | kcal per portion | Carbohydrates (g) | Protein (g) | Total lipids (g) |
|---|---|---|---|---|
| 1.Bacon Krik (São JoãoSabor e Nutrição®) (30 g portion) | 120 | 19.8 | 1.8 | 3.6 |
| 2.White chocolate bonbon (Nestle® Brazil) (20g portion) | 93 | 14 | 0.7 | 3.6 |
| 3.Honey bread (QueroQuero) (30 g portion) | 123 | 28 | 1.6 | 0.4 |
| 4.Salame (Friato®) (50 g portion) | 112 | 2.5 | 6 | 8 |
| 5.Lemon wafer cookies (Parmalat®) (30 g portion) | 165 | 19 | 1.1 | 9.5 |
| 6.Corn flakes (Skiny®) (25 g portion) | 127 | 20 | 1.8 | 4.2 |
| 7.Coated peanuts (Dori®) (25 g portion) | 109 | 20.8 | 1.8 | 2.4 |
| 8.Vanilla mini-cake (Bauducco®) (40 g portion) | 164 | 20 | 2.2 | 8.4 |
| 9.Homemadedulce de leche (Parati®) (30 g portion) | 70 | 17 | 1 | 12 |
| 10. Mini bread rolls (Pullman®) ( 50 g portion) | 154 | 28 | 3.9 | 2.8 |
| 11. Sandwich biscuits of sweet brigadier (Mabel®) (30 g portion) | 147 | 21 | 1.7 | 6.9 |
| 12. Toasted popcorn (São João®) (40 g portion) | 64.4 | 12.4 | 2.1 | 7.5 |
| 13. Cookies (Bauducco®) (30 g portion) | 151 | 19 | 2.1 | 7.5 |
| 14. Maisenabiscuit (Parmalat®) (30 g portion) | 132 | 22 | 2.4 | 3.7 |
| 15. Tapioca flour biscuit (PetaCaseira) (25 g portion) | 117 | 23.17 | 0.9 | 0.93 |
| 16. Pizza flavored biscuit (Miliopã®) (30 g portion) | 128 | 22 | 2.0 | 3.5 |
| 17. Caramel-filled bonbon (Nestlé®) (20 g portion) | 92 | 15 | 0.6 | 3.3 |
| 18. Strawberry sandwich biscuit (Amanda®) (30 g portion) | 125 | 16 | 1 | 4 |
| 19. Cheese flavored chips (Miliopã®) (30 g portion) | 130 | 22 | 2.2 | 3.8 |
| 20. Provolone cheese (Vale Orizona®) (30 g portion) | 103 | 0 | 8.7 | 76 |
| 21. Hot dog sausage (Sadia®) (50 g portion) | 121 | 33.7 | 6.8 | 9.5 |
| 22. Paio sausage (Sadia®) (50 g portion) | 187 | 0 | 8.5 | 17 |
| 23. Bacon flavored chip (Miliopã®) (25 g portion) | 105 | 18 | 1.8 | 2.5 |
| 24. Mini chocolate cake (Bauducco®) (40 g portion) | 164 | 20 | 2.2 | 8.4 |
| 25. Prestígio bonbon (Nestlé®) (18.4 g portion) | 85 | 11 | 0 | 3.8 |
| 26. Mortadella (Friato®) (50 g portion) | 112 | 2.5 | 6 | 8 |
| 27. Smoked Calabrian sausage (Sadia®) (50 g portion) | 182 | 0.8 | 8.7 | 16 |
| 28. Lemon wafer cookies (Amanda®) (30 g portion) | 125 | 16 | 1 | 4 |
| 29. Sugar peanut (Dori®) (15 g portion) | 73 | 8.9 | 1.8 | 3.4 |
| 30. Cream biscuits (Maranata®) (30 g portion) | 190 | 20 | 2 | 5 |
| 31. Chocolate donut (Mabel®) (30 g portion) | 125 | 22 | 1.8 | 3.5 |
| 32. Carrot cake (Ana Maria®) (40 g portion) | 159 | 23 | 2.0 | 5.6 |
| 33. Ready crackling (SaborD’Abadia®) (10 g portion) | 68 | 0 | 2.6 | 6.2 |
| 34. Sweet brigadier (Bauducco®) (30 g portion) | 133 | 18 | 1.7 | 6.0 |
| 35. Baconzitos – bacon chips (Elma Chips®) (25 g portion) | 126 | 15 | 1.9 | 6.7 |
| 36. Cashew (Dunorte®) (15 g portion) | 88 | 3.4 | 3.0 | 6.9 |
| 37. Chicken sausage (Perdigão®) (50 g portion) | 106 | 2.0 | 6.0 | 8.2 |
| 38. Mortadella(Sadilar®) (40 g portion) | 121 | 3.4 | 4.0 | 10 |
| 39. Gomets (Dori®) (20 g portion) | 72 | 18 | 0 | 0 |
| 40. Mini strawberry cake (Bauducco®) (40 g portion) | 146 | 20 | 2.2 | 6.4 |
| 41. Baked cheese chips (Cheetos®) (20 g portion) | 100 | 11 | 0.9 | 4.4 |
| 42. Wavy potato chip (Crony®) (25 g portion) | 153 | 1.4 | 1.7 | 10 |
| 43. Ham (Sadia®) (30 g portion) | 36 | 0.8 | 3.9 | 1.9 |
| 44. Sweet corn flakes (Ki gostoso®) (25 g portion) | 69 | 16 | 1.3 | 0 |
| 45. Peanuts (Paulista®) (15 g portion) | 90 | 13.7 | 1.3 | 0 |
| 46. Bis – chocolate wafer (Lacta®) (30 g portion) | 149 | 19 | 1.8 | 7.1 |
| 47. Passatempo – strawberry filled sandwich biscuit (Nestlé®) (30 g portion) | 135 | 21 | 1.9 | 4.8 |
| 48. Fandangos (Elma Chips®) (22 g portion) | 100 | 16 | 1.5 | 3.5 |
| 49. Teens Bauny – rolls (Marilam®) (30 g portion) | 134 | 20 | 2.4 | 4.8 |
Table 2: Foods offered to the animals of the Cafe groups during the experimental period, with nutritional information.
In addition to palatable foods and standard diet, the animals in the Cafe and Cafe+stress groups received natural water, water with sucrose (at the concentration of 300 g/L, adapted from Estrela et al. [31]) and cola-type beverage ad libitum.
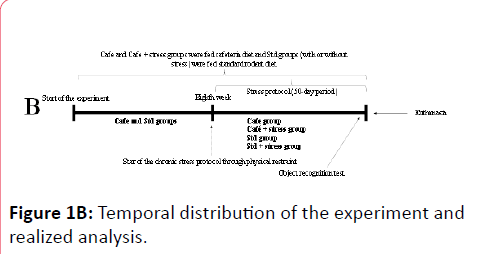
The animals’ body mass was weekly measured using a semianalytical digital scale and an additional evaluation of parameters such as waist circumference and naso-anal length was performed in the eighth experimental week in order to assess whether the animals were obese before the beginning of the stress protocol. After eight experimental weeks (Figure 1B), the Std+stress and Cafe+stress groups were subjected to chronic stress protocol through physical restraint, as it was proposed by Ely et al. [32]. A plastic tube (25 cm x 7 cm) was used to limit the animals’ movements and its front part was perforated in order to allow the animals to breathe. The animals were daily subjected to the stressor for 1 hour (in the afternoon, between 2:00 and 3:00 pm), 5 days a week, during a 50-day period. It is noteworthy that the animals were immobilized in a quiet environment with standard lighting to avoid additional stress or interference in the circadian cycle. The combination between the stress protocol and the cafeteria diet is an attempt to simulate the daily life of obese individuals generally undergoing low-intensity stresses often associated with poor diet.
In the 15th experimental week, the animals were subjected to the object recognition test, which is widely used in trials related to cognitive function involving rodents [33], as described below.
Object recognition test
The object recognition test was carried out in a circular arena (60 cm diameter and 50 cm tall), adapted from Bevins & Besheer [33]. The test was divided into two sessions: a training session, followed by one test session (24 h after training). During the training session, the animals were exposed to two identical objects (in size, form, and color), defined as familiar objects (F1 and F2), for five minutes (square Lego toys). During the test session, a familiar object was replaced by a new object (N) (24 h after training we used a triangular Lego toy), so that the animals could explore a familiar object and a new one for five minutes. At the beginning of each trial the animals were placed in front of the objects, facing the wall, according to Akkerman et al. [34]. The time spent to explore each object was recorded. A crossed-over design was used in all test sessions, so that the new and the familiar objects were placed alternately, in order to exclude potential preference for a certain spatial location of the objects in the box. Exploration was considered smelling and touching objects with the nose or forepaws and when the animal was at a distance equal or less than 2 cm from the objects.The recognition index for each object was calculated for each animal, as described by Pietá- Dias et al. [35], and expressed by the ratio: TOX/(TF+TN) [TOX=time spent exploring the familiar (F) or new (N) object; TF=time spent exploring the familiar object; TN = time spent exploring the new object]. Between sessions, the boxes used in the tests were cleaned with alcohol 10%.
Analyses carried out after the behavioral test
After the behavioral tests were finished, the animals were euthanized by decapitation in order to measure the relative mass of visceral and retroperitoneal fats. To this end, the fat masses were normalized to the body weight using the following formula: fat mass (g)/body weight (g). The relative mass of the adrenal glands was analyzed as stress indicator parameter, according to Estrela et al. [31].
Statistical analysis
The recognition rates were separately calculated for the F and N objects and subjected to variance analysis according to the factorial model (two-way ANOVA), by taking into consideration the factors "nutrition" (standard diet and cafeteria diet) and "condition "(stress and no stress), using 12 animals in each group (using ASSISTAT software, version 7.7 beta - distributed for free). Whenever the F value was significant, we applied the Tukey test at 5% probability for all recognition index comparisons. Each familiar object recognition index was also calculated in the Std group training session, by applying one-sample t test at 50% probability, and using Assistat software, version 7.7 beta. This assessment was particularly important to validate the test. The residual normality was verified using the Shapiro-Wilk test and Bartlett's test was used to check the data homoscedasticity. It is worth highlighting that two trained observers recorded the behavioral parameters during all sessions of the object recognition test. The same video was analyzed twice, resulting in an inter-observer concordance higher than 85%.
Results and Discussion
Physical assessments of the animals
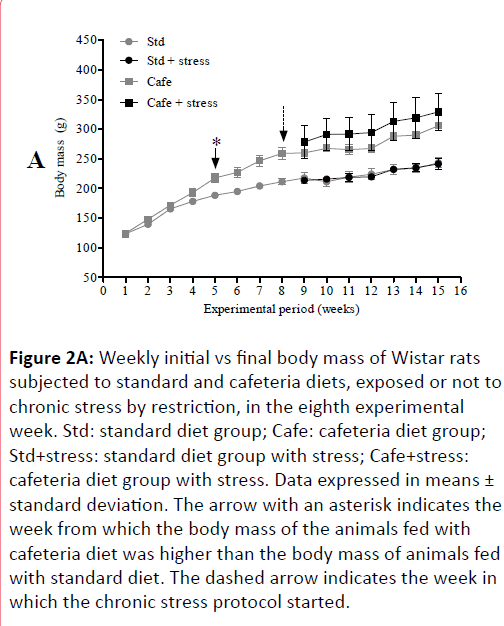
The results of the animals’ physical examination showed that from the 5th experimental week on, the animals fed cafeteria diet showed significant body mass increase in comparison to those fed only standard diet (Figure 2A) and this difference remained until the end of the experiment (Figure 2B). There was no difference between the body masses of stressed and non-stressed animals, indicating that stress had no effect on this parameter.
Figure 2A: Weekly initial vs final body mass of Wistar rats subjected to standard and cafeteria diets, exposed or not to chronic stress by restriction, in the eighth experimental week. Std: standard diet group; Cafe: cafeteria diet group; Std+stress: standard diet group with stress; Cafe+stress: cafeteria diet group with stress. Data expressed in means ± standard deviation. The arrow with an asterisk indicates the week from which the body mass of the animals fed with cafeteria diet was higher than the body mass of animals fed with standard diet. The dashed arrow indicates the week in which the chronic stress protocol started.
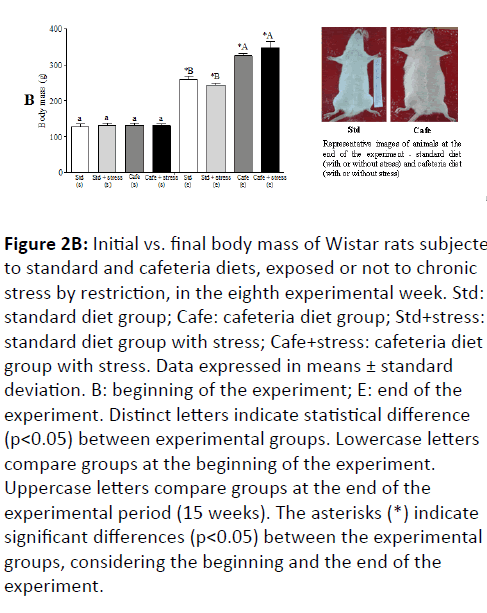
Figure 2B: Initial vs. final body mass of Wistar rats subjected to standard and cafeteria diets, exposed or not to chronic stress by restriction, in the eighth experimental week. Std: standard diet group; Cafe: cafeteria diet group; Std+stress: standard diet group with stress; Cafe+stress: cafeteria diet group with stress. Data expressed in means ± standard deviation. B: beginning of the experiment; E: end of the experiment. Distinct letters indicate statistical difference (p<0.05) between experimental groups. Lowercase letters compare groups at the beginning of the experiment. Uppercase letters compare groups at the end of the experimental period (15 weeks). The asterisks (*) indicate significant differences (p<0.05) between the experimental groups, considering the beginning and the end of the experiment.
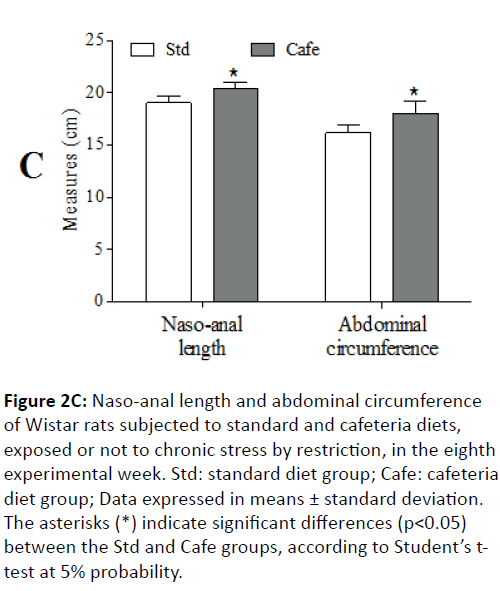
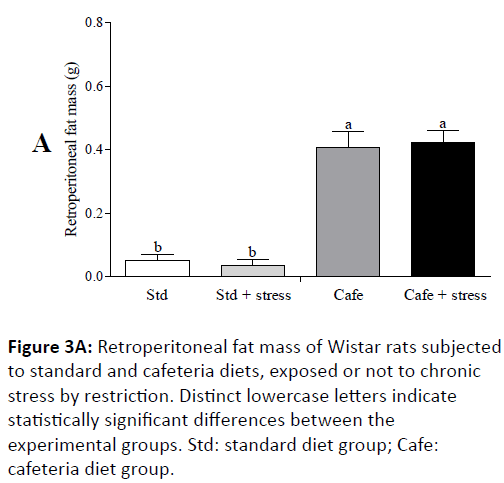
In the 8th experimental week, it was also observed that the animals fed cafeteria diet had higher naso-anal length (p=0.043) and greater waist circumference (p<0.001) (Figure 2C). At the end of the experiment, only the factor “nutrition” had effect on the relative retroperitoneal fat (F(1,44)=71.202; p<0.001) (Figure 3A) and visceral mass (F(1,44)=32.887; p<0.001) (Figure 3B). Figure 3C shows representative images of the visceral fat visual aspect observed in the animals of the Std and Cafe groups (with or without stress). Therefore, these data indicate that the cafeteria diet effectively induced obesity in the animals. The data in the current study corroborate other studies in the literature, which also used cafeteria diet to induce obesity in rodents, and in which the body mass gain was an important obesity predictor [36,37].
Figure 2C: Naso-anal length and abdominal circumference of Wistar rats subjected to standard and cafeteria diets, exposed or not to chronic stress by restriction, in the eighth experimental week. Std: standard diet group; Cafe: cafeteria diet group; Data expressed in means ± standard deviation. The asterisks (*) indicate significant differences (p<0.05) between the Std and Cafe groups, according to Student’s ttest at 5% probability.
Figure 3A: Retroperitoneal fat mass of Wistar rats subjected to standard and cafeteria diets, exposed or not to chronic stress by restriction. Distinct lowercase letters indicate statistically significant differences between the experimental groups. Std: standard diet group; Cafe: cafeteria diet group.
Figure 3C: Representative images of the visual aspect of visceral fat of animals at the end of the experiment - standard diet (with or without stress) and cafeteria diet (with or without stress). Distinct lowercase letters indicate statistically significant differences between the experimental groups. Std: standard diet group; Cafe: cafeteria diet group.
However, the hypothesis that chronic stress could lead animals to a greater obesity status was not confirmed in the current study. Studies, such as that conducted by Bartolomucci et al. [38], showed that chronic stress can elevate the glucocorticoid concentrations, which induce pleasurable and compulsive behaviors such as the intake of sweets and fatty foods.
Therefore, it may increase the abdominal fat deposits, thus taking the body to obesity. Studies performed on humans demonstrate that individuals stressed at work, for example, tend to accumulate large amounts of abdominal fat [39].
On the other hand, animals subjected to hypercaloric diets with high carbohydrates content showed mitigated responses to stress [40]. There is strong evidence that chronic stress can promote energy storage in the abdominal adipose tissue [41], but this finding was not observed in the current study, probably due to stress-inducing protocols have been different.
Macedo [42] evaluated weight and biochemical parameters in male Wistar rats fed with cafeteria diet and subjected to chronic stress and he did not observe the joint influence of stress and obesity on the body mass gain of the animals, on the weight delta (the range between the initial and the final weights), on the Lee index (ratio between the weight and the naso-anal length of the animals) and on the mass of fat tissue per 100 g of body mass.
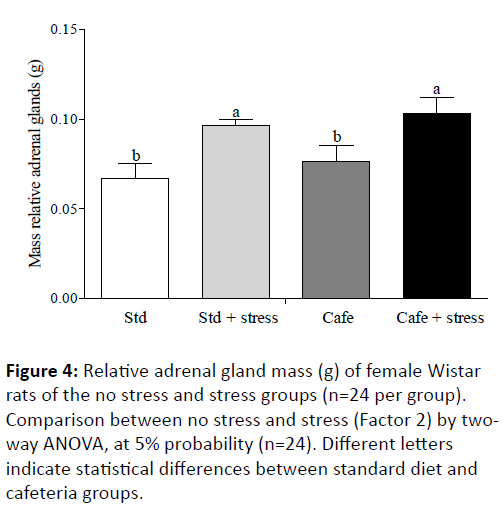
Macedo [42] also advocates that the adrenocorticotropic hormone continuously stimulates the adrenal glands in chronic stress conditions, thus leading these glands to hypertrophy. Thus, since the glucocorticoid concentrations were not evaluated in the present study, the weight of the adrenal glands was used as indirect stress indicator parameter, and it showed that the animals were stressed by the used protocol. The effect of the factor 2 "condition" (exposure to stress) was observed in the adrenal glands relative mass (F(1,44)=6.681,p=0.032), showing the hypertrophy of these glands in stressed animals in comparison to non-stressed animals (Figure 4).
Figure 4: Relative adrenal gland mass (g) of female Wistar rats of the no stress and stress groups (n=24 per group). Comparison between no stress and stress (Factor 2) by twoway ANOVA, at 5% probability (n=24). Different letters indicate statistical differences between standard diet and cafeteria groups.
This result corroborates previous studies that used protocols similar to that used in the current study and showed that the exposure to stress by daily restraint may cause adrenal glands hypertrophy [31,43].
It is worth emphasizing that the measurement of glucocorticoid concentrations may not indicate that the animals are stressed. It is well known that repeated exposure to the same stressor can lead to habituation of the hypothalamic-pituitary-adrenal (HPA) axis [44]. Therefore, the animals subjected to chronic stress did not exhibit the same behavior and did not experience the same consequences the animals exposed to acute stress did [45].
Torres et al. [44] evaluated adult male Wistar rats and found that the corticosterone concentrations increased after the exposure to acute restraint. In contrast, Macedo et al. [46] showed no changes in corticosterone concentrations after repeated chronic stress (for 6 weeks), thus suggesting HPA axis adaptation. Importantly, these studies used male Wistar rats, whereas the current study used female rats. Macedo et al. [47] showed increased weight in the adrenal glands of male Wistar rats subjected to 12 weeks of restraint-induced chronic stress, thus corroborating the results found in the current study.
Balog et al. [27] recently evaluated biochemical markers related to cardiometabolic risks as well as leptin and sex hormone receptors in the adrenal glands of male, ovariectomized (OVX) and non-ovariectomized (non-OVX) female rats (Sprague-Dawley) under chronic stress. Early changes in the specific gender pattern were found in the adrenal glands in response to stress. Chronic stress changes on the adrenal glands were related to the decreased testosterone receptor in females and to the increased estrogen receptor in males. Thus, additional studies are needed to help better understanding the hormonal dynamics in stressed Wistar rats fed with cafeteria diet and its effects on the glucocorticoid concentrations, as well as on the adrenal glands’ structure and function.
Behavioral assessment
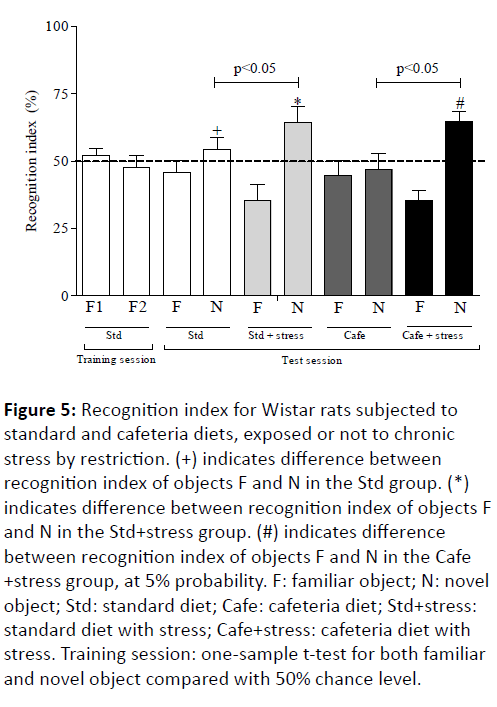
Our data initially showed that the recognition rates of familiar objects (F1 and F2) in the animal training session of the control group were different from zero and showed no significant differences according to the one-sample t-test, at 50% chance level (Figure 5).
Figure 5: Recognition index for Wistar rats subjected to standard and cafeteria diets, exposed or not to chronic stress by restriction. (+) indicates difference between recognition index of objects F and N in the Std group. (*) indicates difference between recognition index of objects F and N in the Std+stress group. (#) indicates difference between recognition index of objects F and N in the Cafe +stress group, at 5% probability. F: familiar object; N: novel object; Std: standard diet; Cafe: cafeteria diet; Std+stress: standard diet with stress; Cafe+stress: cafeteria diet with stress. Training session: one-sample t-test for both familiar and novel object compared with 50% chance level.
This result constitutes one of the test validation indicators, since it shows that the random scanning of the objects in the training session resulted in an equal holding of both objects, and excludes potential influences in the preference for a particular spatial location of objects placed in the test arena. On the other hand, the object recognition test revealed significant differences between the familiar and new objects (F and N) recognition indices in most experimental groups (Figure 5). The animals in the Std, Std+stress and Cafe+stress groups showed increased N object recognition index (p<0.001) in comparison to the F object. This result was not observed in the Cafe group rats (Figure 5), and it suggests that obesity has adversely affected the animals’ cognitive function, thus corroborating the results already observed in other study [12]. Therefore, it is suggested that obesity alone has interrupted the hippocampal-dependent memory consolidation.
Our data also showed memory improvement in the stressed animals (obese and non-obese). Figure 5 shows higher N object recognition index in the Std+stress and Cafe+stress groups, in comparison to their respective controls. These data corroborate other studies that have shown the effects of moderate stress on the memory of experimental models [48,49].
The neural mechanisms responsible for the improvement in cognitive function after exposure to chronic moderate stress (obese or non-obese) have not been investigated in the current study. The improved memory in the Cafe+stress group may be particularly related to glutamatergic transmission in the prefrontal cortex (a key brain region controlling cognition and emotion) facilitating working memory and not related to lesions in the hippocampus or destruction of inputs to the hippocampus disrupts acquisition of the object recognition test, as observed in other studies [50,51]. According to Joëls [30], the stress within the context of a learning situation leads to the release of corticosteroids, resulting in focused attention and memory improvements. In this case, the deleterious effect of obesity on the animals’ memory may have been modulated by stress conditions imposed to them, which led to improvement in the cognitive function of obese animals.
Thus, the herein presented study supports the hypothesis that chronic exposure to moderate and repeated stress improves the memory of obese female Wistar rats; this aspect has never been demonstrated before. Yuen et al. [48] studied rodents and observed that acute stress can produce a beneficial effect on learning and on memory due to the effect of the stress hormone corticosterone on the prefrontal cortex of the brain, which is a key region that controls learning and emotion. In another study, Costa et al. [49] suggested that the manipulation of rats (which would be a stressful situation) reduces anxiety levels and improves learning and memory skills.
Despite our evidence, it is important to note that the restraint-induced stress leads to neuronal loss and to morphological remodeling in the dorsal hippocampus [52], as well as to the dendritic remodeling of neurons in the prefrontal cortex and amygdala of male rodents [53]. Furthermore, it was observed that the chronic restraint of male rats also causes the atrophy of the dendritic branches in the spine and changes the synaptic communication between these regions [54]. As for female rats, Galea et al. [55] also demonstrated that constraint-induced stress also produces moderate atrophy of the hippocampal neurons, thus suggesting memory deficit in the animals. Although this evidence converge to the fact that stress causes cognitive impairments in animals, it is necessary to take into consideration that the aforementioned studies used different stress-inducing protocols from that used in the current study. In addition, different rodent strains with different ages were also used, and these aspects may explain the differences between these studies and the present one.
Although the corticosterone concentrations were not evaluated in the present study, it is well established that the adrenal medulla (epinephrine) and adrenal cortex (corticosterone in rats, cortisol in humans) hormones are released during and immediately after the stressful stimulus. Therefore, the activation degree of these hormonal systems depends on the severity and on the type of stressor [56]. Thus, a possible explanation for the lack of harmful effects on the memory of stressed female Wistar rats could be related to the type of stress-inducing protocol used in the animals. Since the herein used protocol was a moderate stress protocol, although repetitive, it is possible that the animals’ body developed adaptations that might have prevented cognitive deficits in animals. Another explanation would be related to the estrous cycle stage of the studied females the day the behavioral test was performed. This explanation may be extrapolated to the lack of cognitive impairments in non-stressed obese females. Although the non-determination of the estrous cycle stage of the female rats is a limitation in the current study, it is possible that the evaluated females did not show synchronization in their estrous cycle, i.e., some of them showed hormonal peaks and the others did not. It is well known in the literature that the hormone peaks observed at certain estrous cycle stages in females significantly influence the animals’ behavior [57].
Finally, other investigations should be conducted aiming at better understanding the cognitive and physiological aspects of the obesity-stress association in different ovarian cycle phases. As for stress, Luine et al. [58] conducted a study on rats and reported neural and behavioral changes dependent on the animals’ gender, chronic stress duration and age. Moreover, the change in the estradiol levels between the genders throughout life appears to contribute to their different responses to stress [59]. Thus, the stress theories dependent on the central nervous system modulations–which were solely developed in male models, focused on peripheral physiological processes and tested in adults-may require revision when they are applied to a more diverse population (in terms of age and gender), at least in relation to the cognition and anxiety neural functions. In addition, the results of the current study suggest that other stressors and neural functions should be investigated to determine whether age, gender and gonadal hormones also have some influence on studies jointly involving obesity and chronic stress.
Conclusion
It is concluded that the cafeteria diet used in the current study effectively induced obesity in female Wistar rats. In addition, the restraint-related chronic stress protocol successfully induced stress in the animals. The chronic stress induced in the animals did not increase obesity in the selected female rodent lineage. Finally, the hypothesis that stress in obesity (established in the experimental conditions of this study) could cause positive effects on the long-term memory of female Wistar rats was confirmed. It is strongly suggested that the mechanisms involving the effect of chronic-stress in obesity should be better investigated in female rats, given the organic complexity involved in these modern diseases. In addition, it is important to emphasize that different stress protocol and stress type can have different outcomes.
References
- Lancet T (2006) Curbing the obesity epidemic. Lancet 367: 1549.
- Haslam DW, James WPT (2005) Obesity. Lancet 366: 1197-1209.
- Lakhan SE, Kirchgessner (2013) The emerging role of dietary fructose in obesity and cognitive decline. Nutr J 12: 114.
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, et al. (2006) Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry 48: 57-61.
- Gunstad J, Strain G, Devlin MJ, Wing R, Cohen RA, et al (2011) Improved memory function 12 weeks after bariatric surgery. SurgObesRelat Dis 7: 465-472.
- Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, et al. (2014) Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg 207: 870-876.
- Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, et al. (2014) Improved memory function two years after bariatric surgery. Obes 22: 32-38.
- Davidson TL, Tracy AL, Schier LA, Swithers SE (2014) A view of obesity as a learning and memory disorder. J ExpPsycholAnim Learn Cogn 40: 261-279.
- Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, et al. (2012) Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem 98: 25-32.
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, et al (2008) Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology 149: 2628-2636.
- Valladolid-Acebes I, Stucchi P, Cano V, Fernandez-Alfonso MS, Merino B, et al. (2011) High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol Learn Mem 95: 80-85.
- Camer D, Yu Y, Szabo A, Fernandez F, Dinh CH, et al. (2015) Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. ProgNeuropsychopharmacolBiol Psychiatry 59: 68-75.
- Greenwood CE, Winocur G (1996) Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. BehavNeurosci 110: 451-459.
- Winocur G, Greenwood CE (2005) Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging 26: 46-49.
- Monroe SM (2008) Modern Approaches to Conceptualizing and Measuring Human Life Stress. Annu Rev ClinPsychol 4: 33-52.
- Taylor SE, Stanton AL (2007) Coping resources, coping processes, and mental health. Annu Rev ClinPsychol 3: 377-401.
- Patrick JH, Stahl ST, Sundaram M (2011) Disordered eating and psychological distress among adults. Int J Aging Hum Dev 73: 209-26.
- Feldman S, Weidenfeld J (1999) Glucocorticoid receptor antagonists in the hippocampus modify the negative feedback following neural stimuli. Brain Res 821: 33-37.
- Bowman RE, Beck KD, Luine VN (2003) Chronic stress effects on memory: sex differences in performance and monoaminergic activity. HormBehav 43: 48-59.
- Davidson TL, Tracy AL, Schier LA, Swithers SE (2014) A view of obesity as a learning and memory disorder. J ExpPsycholAnim Learn Cogn 40: 261-279.
- Sood V, Chakravarti RN (1976) Systemic stress in the production of cardiac thrombosis in hypercholesterolaemic rats. Res Exp Med 67: 31-45.
- Yamaguchi K, Goko H, Matsuoka A (1979) Effects of electric stress on glucose metabolism, glucose-stimulated cyclic adenosine 3',5'-monophosphate accumulation and 45 Ca++ efflux in isolated pancreatic islets from rats fed with a high fat diet. EndocrinolJpn 26: 549–557.
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, et al. (2005) Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport 16: 39-43.
- Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, et al. (2009) Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behavioural Brain Research 204: 117-123.
- Ng M, Fleming TD, Biryukov S (2014) Global, regional and national prevalence of overweight and obesity in children and adults 1980-2013: A systematic analysis. The Lancet 14: 60460-60468.
- Asarian L, Geary N (2013) Sex differences in the physiology of eating. Am J PhysiolRegulIntegr Comp Physiol. 305: R1215-1267.
- Balog M, Miljanovic M, BlaÃÆââ¬Â¦Ãâþetic S, Labak I, Ivic V, et al. (2015) Sex-specific chronic stress response at the level of adrenal gland modified sexual hormone and leptin receptors. Croat Med J. 56: 104-112.
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS (1996) Restraint stress reversible enhances spatial memory performance. PhysiolBehav 59: 27-32.
- Diamond DM, Bennett MC, Fleshner M, Rose GM (1992) Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus 2: 421–430.
- Joëls M (2006) Corticosteroid effects in the brain: U-shape it. Trends PharmacolSci 27: 244–250.
- Estrela DC, Silva WAM, Guimarães ATB, Mendes BO, Castro ALS, et al (2015) Predictive behaviors for anxiety and depression in female Wistar rats subjected to cafeteria and stress. Physiol&Behav 151: 252-263.
- Ely DR, Dapper V, Marasca J, Corrêa JB, Gamaro GD, et al. (1997) Effect of restraint stress on feeding behavior of rats. PhysiolBehav 61: 395-398.
- Bevins R, Besher J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory”. Nature Protocols 1: 1306-1311.
- Akkerman S, Blokland A, Reneerkens O, van Goethem NP, Bollen E, et al. (2012) Object recognition testing: methodological considerations on exploration and discrimination measures. Behavioural Brain Research 232: 335-347.
- Pietá-Dias C, Martins de Lima MN, Presti-Torres J, Dornelles A, Garcia VA, et al. (2007) Memantinereduzces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscienc 46: 1719-1725.
- Mendes FCV, Moreira BMT, Marsiglio GN, Carabelli B, Munhoz AC, et al. (2013) Dieta de cafeteria remodela a estrutura da aorta de ratosobesos. Sabios: Rev SaúdeBiol 8: 85-91.
- Warneke W, Klaus S, Fink H, Langley-Evans SC, Voigt JP (2014) The impact of cafeteria diet feeding on physiology and anxiety-related behaviour in male and female Sprague–Dawley rats of different ages. PharmacolBioch and Behav 116: 45-54.
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, et al. (2009) Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PloS ONE 4: e4331.
- Kouvonen A, Kivimaki M, Cox SJ, Cox T, Vahtera J (2005) Relationship between work stress and body mass index among female and male employees. Psychosom Med 67: 577-583.
- Pecoraro N, Gomez F, Bhargava A, Dallman MF (2004) Chronic stress promotes palatable feeding, wich reduces signs of stress: feedforward and feedback effects of chronic stress. Endocronology 145: 3754-3762.
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, et al. (2006) Glucocorticoids, chronic stress and obesity. Prog Brain Res 153:75-105.
- Macedo IC (2010) Estressecrônicoassociado à dietahipercalóricaemratosWistar: parâmetrosponderais e bioquímicos. Dissertação (MestradoemFisiologia) 111.
- Bloss EB, Janssen WG, McEwen BS, Morrison JH (2010) Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosc 30: 6726-6731.
- Torres ILS, Gamaro GD, Silveira-Cucco SN, Michalowski MB, Corrêa JB, et al. (2001) Effect of acute and repeated restraint stress on glucose oxidation to CO2 in hippocampal and cerebral cortex slices. Braz J Med Biol Res 34: 111-116.
- Lachuer J, Delton I, Buda M, Tappaz M (1994) The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res 638: 196-202.
- Macedo IC, Medeiros LF, Rozisky JR, Laste G, Souza VS, et al. (2012) Cafeteria diet-induced obesity plus chronic stress alter serum leptin levels. Peptides 38: 189-196.
- Macedo IC, Rozisky JR, Oliveira C, Oliveira CM, Laste G, et al. (2015) Chronic stress associated with hypercaloric diet changes the hippocampal BDNF levels in male Wistar rats. Neuropeptides 51: 75–81.
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, et al. (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. PNAS 106: 14075-14079.
- Costa R, Tamascia ML, Nogueira MD, Casarini DE, Marcondes FK (2012) Handling of Adolescent Rats Improves Learning and Memory and Decreases Anxiety. J Am Assoc Lab AnimSci 51: 548–553.
- McEwen BS (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22: 105-122.
- Elizalde N, Gil-Bea FJ, Ramírez J, Aisa B, Lasheras B, et al. (2008) Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: effect of antidepressant treatment. Psychopharmacology 199: 1-14.
- Christian KM, Miracle AD, Wellman CL, Nakazawa K (2011) Chronic stress-induced hippocampal dendritic retraction requires CA3 NMDA receptors. Neuroscience 174: 26-36.
- Hosseini-sharifabad M, Hadinedoushan H (2007) Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. AnatSciInt 82: 211-217.
- Krugers HJ, Goltstein PM, van der Linden S, Joe¨ls M. (2006) Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci 23: 3051-3055.
- Galea LA, McEwen BS, Tanapat P, Deak T, Spence RL, et al. (1997) Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81: 689-697.
- Korte SM (2001) Corticosteriods in relation to fear anxiety and psychopathology. NeurosciBiobehav Rev 25: 117-142.
- Byers SL, Wiles MV, Dunn SL, Taft RA (2012) Mouse Estrous Cycle Identification Tool and Images. PLoS ONE 7: e35538.
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ (2007) Chronic stress and neural function: accounting for sex and age. J Neuroendocrinol 19: 743-751.
- Luine VN (2014) Estradiol and cognitive function: past, present and future. HormBehav 66: 602-618.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences